The many roles surfactants play in cosmetics, can be quite confusing. They are used for a variety of different reasons and often, in conjunction with one another. This post will explain what surfactants are, their different roles, as well as the four different types.
Table of Contents
What is a surfactant?
The technical name for them is surface active agent. The abbreviation “surfactant” comes from their technical name: SURFace ACTive AgeNT.
Surfactants hold many different roles; they can be found in a vast array of products from toothpaste to shampoo.
Let’s look at some of the reason why we use surfactants:
Detergents / Cleansing
Detergents and cleansers are useful to remove dirt and oil. Water is able to remove some dirt, but it can’t remove the oil. Oil the body naturally produces is called sebum.
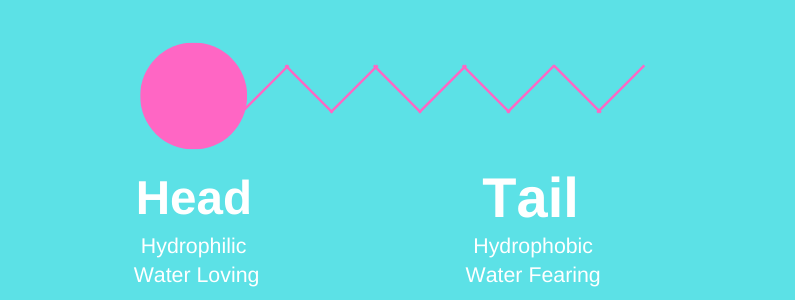
Surfactants have a head that is hydrophilic (attracted to water) and a tail that is hydrophilic (water fearing).
Meaning that the surfactants grab onto the dirt and oil with their tails and grab onto water with their head. This allows you to easily rinse the dirt and oil off and down the drain with the water.
Dispersing Agents
This means that they are able to improve the separation of particles to prevent them clumping together or settling.
Emulsifiers
Combine two incompatible ingredients (think water and oil) together in a formula. See my Emulsions post for more details.
Foaming Agents
Increase foam in a product, think shampoo and bubble baths.
It is important to note, that more foam does not always mean more cleaning abilities. However, many consumers will associate foam with cleansing, so the foam is important in a finalized product
Wetting Agents
To spread a product, so it does not stay together. The wetting agent will lower the surface tension, to make something more effective at penetrating the surface or more effective at spreading.
For example: If you were to put water into a spray bottle and spray it on a window, the water would naturally bead together. This is because the water is attracted to water (like attracts like). However, to clean the window, you want it to spread evenly, not bead together. Hence, the importance of wetting agents.
Thickening
When your formula is mostly water, surfactants will form into micelles. There circle structures (micelles) are used to create emulsions and to thicken.

Right now, you have to be thinking “Wait, how do the micelles thicken it?”
This depends on how closely the micelles are packed together. The closer together the micelles are, the thicker the product and vise versa.
The charge of your micelles can cause them to repel one another. The charge can be lowered by adding salt. This will minimize the charge and allowing the micelles to get closer together. Which in turn, thickens the product.
Types of Surfactants and Their Uses:
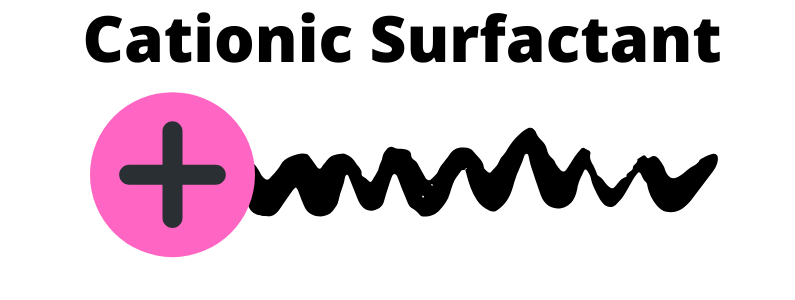
Cationic
Positively charged which makes it good at bonding to dirt and grease. They do not clean very well, so they are typically not used to clean. Positively charged surfactants are better suited to condition.
These are best used when foaming is not necessary. Since the positive charge gives the cationic surfactant a role that is skin loving and conditioning.
The positive charge is attracted to the negative change of our hair. This is why cationic surfactants are commonly used in hair care conditioner. Meaning it sticks to hair (even after rinsing) to reduce static.
Works well with nonionic and amphoteric surfactants. The positive and negative charges do not allow them to be combined with anionic surfactants. This means that cationic surfactants are not compatible with anionic surfactants.
Uses: Conditioner, anti-static spray and toothpaste
Example: Cetylpyridinium chloride (CPC)
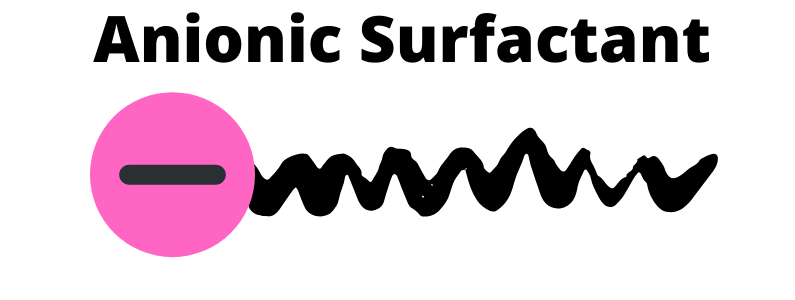
Anionic
Anionic surfactants are the most commonly used of all the surfactants. These surfactants have a negative charge and have a water loving head. Anionic surfactants have a great foaming properties, cleansing and washing abilities and they are great at removing oil. Plus, they are relatively inexpensive.
Does not work as good with hard water. Since they are so good at cleaning, they can also cause some skin irritation. It is normally combine with amphoteric surfactants. Typically, the anionic surfactants will need to be combine with another surfactant to maintain optimal use and to reduce skin irritation.
Uses: Shampoo and laundry soap
Example: Sodium Lauryl Sulfoacetate (SLSa)
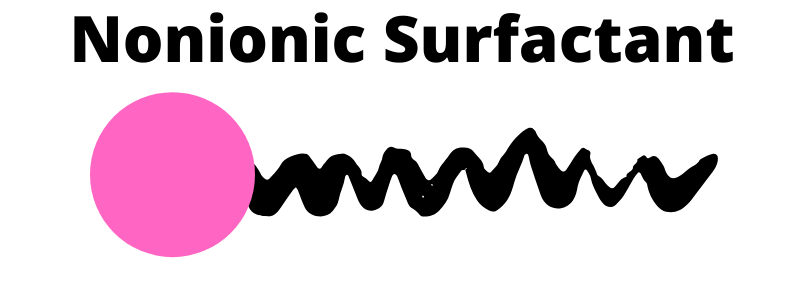
Nonionic
Nonionic, as the name suggests, has no charge. It works great with hard water and is great at removing oil. Salt has no thickening effect on nonionic surfactants as they have no charge. They have no foaming capabilities. On the flip side, nonionic surfactants do give a mild cleansing.
They can be ethoxylated when combined with ethylene oxide. And in simple terms that means, that the water loving heads start to love water even more… making them a solubilizer.
Good at enhancing foam and making a finished product more mild. Often used as an emollient.
Compatible with all other surfactants.
Uses: Solubilizer and baby shampoo
Example: Decyl glucoside and polysorbate 20

Amphoteric
Amphoteric surfactants can be any sign. The sign is determined by the ph of the product.
Super mild cleaner.
Low PH
When the final product has a low ph level, the amphoteric surfactant takes on a nourishing and conditioning role.
High PH
When the final product has a high ph level, the amphoteric surfactant takes on a high foaming and cleansing role. Similar to Anionic surfactant.
Amphoteric surfactants are versatile, meaning that they can be used in conjunction with any type of surfactant. Or, they can be used alone. Amphoteric surfactants can be in a blend with anionic surfactants, making the end product more mild. They can also used to thicken a product and to control bubbles. This can make smaller creamy bubbles. They typically do not foam well on their own and they can be expensive.
Uses: Bath and body and hair care products.
Example: Coco Betaine

5 thoughts on “Surfactants Explained”
Waoo thank you so much for this explain I do can understand much better 🌷 Merry Christmas 🎁🎄
Comments are closed.